Categories
Essays
Links
Author Archives: James Carroll
LumiThera Receives Notice of Award for $2.5 Million National Eye Institute Grant to Support U.S. Multi-Center Clinical Trial for Treating Dry Age-Related Macular Degeneration
LumiThera Inc., a commercial stage medical device company creating a photobiomodulation (PBM) treatment for ocular disorders and disease, today announced it is a recipient of a small business innovative research (SBIR) phase II grant from the National Institute of Health (NIH) and the division of the National Eye Institute (NEI).
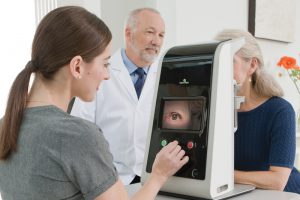 The phase II grant supports a prospective, randomized, multi-center human clinical trial in U.S. subjects diagnosed with dry age-related macular degeneration (dry AMD.) The LIGHTSITE III trial, which is subject to FDA Investigational Device Exemption (IDE) approval, will test vision and examine disease pathology in the eye following PBM treatments using the Company’s Valeda™ Light Delivery System. Subjects will be followed for up to two years. In 2018, LumiThera obtained a CE mark to commercialize the Valeda™ Light Delivery … Continue reading
The phase II grant supports a prospective, randomized, multi-center human clinical trial in U.S. subjects diagnosed with dry age-related macular degeneration (dry AMD.) The LIGHTSITE III trial, which is subject to FDA Investigational Device Exemption (IDE) approval, will test vision and examine disease pathology in the eye following PBM treatments using the Company’s Valeda™ Light Delivery System. Subjects will be followed for up to two years. In 2018, LumiThera obtained a CE mark to commercialize the Valeda™ Light Delivery … Continue reading
Posted in Industry
on LumiThera Receives Notice of Award for $2.5 Million National Eye Institute Grant to Support U.S. Multi-Center Clinical Trial for Treating Dry Age-Related Macular Degeneration
New Technologies, Treatments Can End Opioid Use
 Those suffering from America’s opioid crisis recently received two major messages of hope.
Those suffering from America’s opioid crisis recently received two major messages of hope.
On Oct. 24, President Donald Trump signed the Opioid Crisis Response Act (OCRA) into law. Dozens of bills designed to address the diverse aspects of the opioid crisis were consolidated into one strategic and integrated approach.
OCRA received overwhelming bi-partisan support in both Chambers.
HR6, which is now Public Law 115-2716, broke new ground in being the first legislation to mandate aggressive development and adoption of alternative pain treatments that include “innovative medical technologies for pain management.”
On Oct. 11, Congress held its first ever briefing on ending opioid use through “innovative medical technologies for pain management.”
Photobiomodulation (PBM) was the featured technology.
Read the full news story on newsmax.com
Posted in Industry, THE FUTURE OF PBM/LLLT
on New Technologies, Treatments Can End Opioid Use
LumiThera and Optos Announce Collaboration to Commercialize the Valeda™ Light Delivery System for Treating Dry Age-Related Macular Degeneration in Europe
 LumiThera Inc., a commercial-stage medical device company focused on developing photobiomodulation (PBM) therapies for ocular disorders and disease, announced a distribution agreement with Optos Plc, a division of Nikon, Inc., Japan to exclusively distribute the Valeda™ Light Delivery System for the treatment of dry age-related macular degeneration (AMD) in 12 European countries. Optos is recognized as the leading provider of ultra-widefield retinal imaging devices to eyecare professionals for improved patient eye care. The announcement follows the recent CE Mark Certification for the European Union (EU) for the treatment of dry AMD utilizing LumiThera’s Valeda™ Light Delivery System.
LumiThera Inc., a commercial-stage medical device company focused on developing photobiomodulation (PBM) therapies for ocular disorders and disease, announced a distribution agreement with Optos Plc, a division of Nikon, Inc., Japan to exclusively distribute the Valeda™ Light Delivery System for the treatment of dry age-related macular degeneration (AMD) in 12 European countries. Optos is recognized as the leading provider of ultra-widefield retinal imaging devices to eyecare professionals for improved patient eye care. The announcement follows the recent CE Mark Certification for the European Union (EU) for the treatment of dry AMD utilizing LumiThera’s Valeda™ Light Delivery System.
“The Distribution agreement with Optos allows LumiThera to begin commercialization throughout Europe and establishes a collaboration with a partner in the retinal imaging area,” stated Clark Tedford, Ph.D., LumiThera President and CEO. “Optos is a global leader in ultra-widefield retinal imaging technologies and we are honored to be working with them to open up a new age in the treatment of … Continue reading
Posted in Industry
on LumiThera and Optos Announce Collaboration to Commercialize the Valeda™ Light Delivery System for Treating Dry Age-Related Macular Degeneration in Europe
LumiThera, Inc. to Debut Valeda Light Delivery System at the 18th Congress of the EURETINA
 LumiThera® Inc., a commercial-stage medical device company focused on developing photobiomodulation (PBM) therapies for ocular disorders and diseases, announced that it will participate in the combined exhibition of the 18th EURETINA Annual Meeting and the 36th Congress of the European Society of Cataract and Refractive Surgeons (ESCRS), held in Vienna, Austria from the 20th to the 26th of September 2018.
LumiThera® Inc., a commercial-stage medical device company focused on developing photobiomodulation (PBM) therapies for ocular disorders and diseases, announced that it will participate in the combined exhibition of the 18th EURETINA Annual Meeting and the 36th Congress of the European Society of Cataract and Refractive Surgeons (ESCRS), held in Vienna, Austria from the 20th to the 26th of September 2018.
EURETINA actively promotes new diagnostic developments, advances in vitreoretinal surgery, the development and application of new drugs, and changes in the treatment of macular degeneration.
LumiThera will debut the Valeda™ Light Delivery System for the treatment of dry AMD in the exhibition hall and will host a symposium on PBM technology entitled, “Photobiomodulation: An Innovative, Mitochondria-targeted Therapy for Dry AMD and Other Ocular Diseases” on September 20th.
Read the full press release:
LumiThera, Inc. to Debut … Continue reading
Posted in Industry
on LumiThera, Inc. to Debut Valeda Light Delivery System at the 18th Congress of the EURETINA
PBM Therapy Literature Watch February 2018
36 Photobiomodulation therapy papers published in February 2018. Highlights include:
- DNA repair mechanisms, modulation of telomere maintenance
- Improved motor response in patients with spinal cord injury
- Prevention of < grade 2 radiodermatitis in breast cancer patients (RCT)
- No adverse effects on SCC primary cancer, recurrence or survival
- Improved physiological and performance parameters in runners
- Improved depth of anaesthesia during endodontic treatment
Posted in Research
on PBM Therapy Literature Watch February 2018
LumiThera LT-300 Device for treating Dry Advanced Macular Degeneration granted CE Mark
 “The CE mark allows LumiThera to begin commercialization throughout the 28 EU member states and coincides with the initiation of the LIGHTSITE II Clinical Study in select European sites in the upcoming months,” stated Clark Tedford, Ph.D., LumiThera President and CEO. “We are excited to be able to offer a safe and effective early stage clinical intervention for patients with dry AMD.”
“The CE mark allows LumiThera to begin commercialization throughout the 28 EU member states and coincides with the initiation of the LIGHTSITE II Clinical Study in select European sites in the upcoming months,” stated Clark Tedford, Ph.D., LumiThera President and CEO. “We are excited to be able to offer a safe and effective early stage clinical intervention for patients with dry AMD.”
“It is very exciting to see the development of PBM treatment for dry AMD patients,” stated Samuel Markowitz, M.D., Department of Ophthalmology and Vision Sciences, University of Toronto. “These patients have limited options and losing their central vision is horribly debilitating to their quality of life. The previous LIGHTSITE I Clinical Studies demonstrated that PBM therapy was most beneficial in early stage dry AMD patients. It was also determined that retreatments at scheduled intervals will be needed to maintain clinical benefits.”
Read the full press release:
LumiThera LT-300 Device For Treating Dry … Continue reading
Posted in Industry
on LumiThera LT-300 Device for treating Dry Advanced Macular Degeneration granted CE Mark
PBM Therapy Literature Watch January 2018
27 Photobiomodulation therapy papers published in January 2018. Highlights include:
- Treatment 6h before + immediately before exercise best for reducing muscle fatigue
- Review of pre-clinical data for dementia, Parkinson’s, stroke, trauma and depression
- Improved bacteriological, cytological and clinical benefits after root planing (RCT)
- Improved wound healing of skin graft donor site (RCT)
- Systematic review of RCTs on LED PBM therapy in dermatology
- NSAIDs vs PBM Therapy post root canal (RCT)
- ESWT vs PBM Therapy for Plantar Fasciitis (RCT)
- Effect of PBM on cerebral hemodynamics and metabolism are not thermal
Posted in Research
on PBM Therapy Literature Watch January 2018
 Featured Testimonials
Featured Testimonials