Categories
Essays
Links
Category Archives: Industry
Making the hardest days easier – photobiomodulation for treatment of oral mucositis
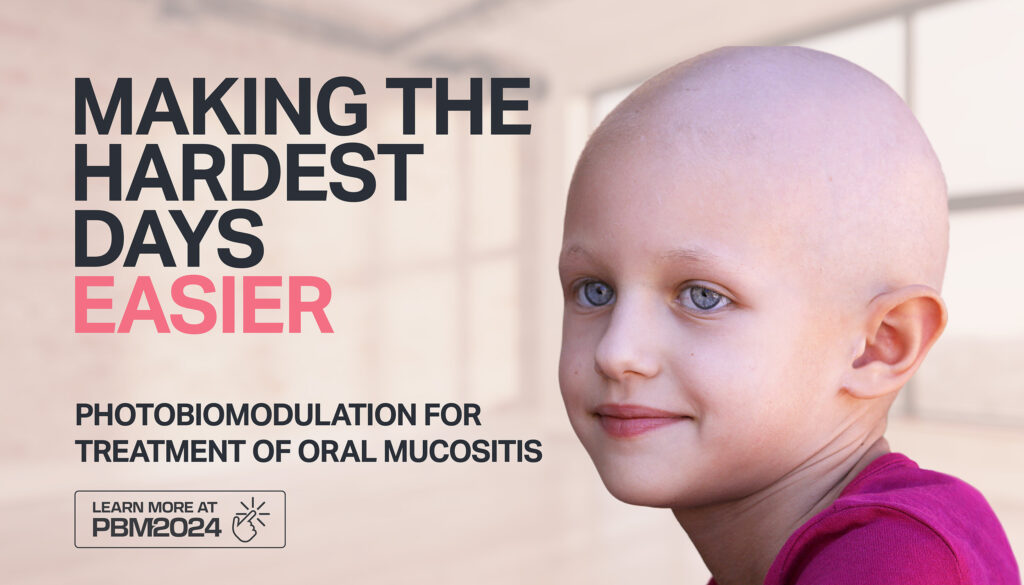
Oral mucositis is a common side effect of high-dose chemotherapy or radiotherapy, leaving many patients unable to eat, drink, or swallow and consequently requiring tube feeding.
Until recently, there had been no satisfactory treatment or method of prevention. However, Photobiomodulation (PBM), a light therapy treatment proven safe and effective by the National Institute for Health and Care Excellence (NICE), is now widely used across the NHS with incredible results.
Jenny Gale, a head and neck clinical nurse specialist at East Suffolk and North Essex NHS Trust, said: “It can really make your mouth sore, and it hurts to swallow, which can make eating and drinking difficult, as well as impacting your general wellbeing.”
She added: “PBM is completely painless and very simple to carry out; most patients can … Continue reading
Posted in Industry, PBM/LLLT, THE FUTURE OF PBM/LLLT
on Making the hardest days easier – photobiomodulation for treatment of oral mucositis
I was alive, but now I’m living again, says cancer survivor Cindy
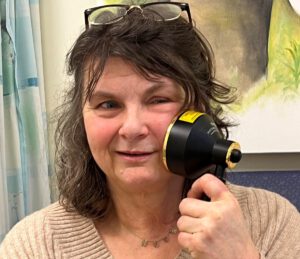 Cancer survivors are getting their lives back – thanks to a trail-blazing initiative at a bespoke post-cancer service at Nottingham City Hospital.
Cancer survivors are getting their lives back – thanks to a trail-blazing initiative at a bespoke post-cancer service at Nottingham City Hospital.
Nottingham Macmillan Late Effects helps people manage the physical, the psychological, and the psychosexual consequences of cancer treatment – and is the only place in the UK using photobiomodulation (PBM) therapy to treat the long-term effects of radiotherapy.
Cindy Martin, 64, who was left with disfiguring scars after surgery for stage 4 cancer of the saliva gland, says PBM therapy has given her back her life.
“The radiotherapy was so intense, my skin became necrotic. By the time I found Late Effects, I was convinced I would always have open wounds on my face.”
Radiation-induced fibrosis – or tissue scarring – is completely unavoidable and can develop in the area being treated with radiotherapy years after the radiotherapy has stopped – with life-changing effects on patients.
“Looking through photographs from … Continue reading
Posted in Industry, Testimonials, THE FUTURE OF PBM/LLLT
on I was alive, but now I’m living again, says cancer survivor Cindy
THOR and PBM making national news
The BBC and Daily Mail are helping to reveal PBM (Photobiomodulation Therapy) into the wider community. With more public awareness of the amazing benefits how PBM light therapy is massively improving lives, we hope it will encourage more people to search it out and ask for this treatment in their own locations.
Read the BBC article:
https://www.bbc.com/news/articles/cg3ee8xl96eo
Read the Daily Mail PDF article:
Daily Mail Gadget that shines bright red LEDs to mend cancer scars now being used to treat debilitating scarring and swelling in the mouth caused by radiotherapy
Posted in Industry, PBM/LLLT, THE FUTURE OF PBM/LLLT
on THOR and PBM making national news
New light therapy helps to ease pain for head and neck cancer patients
Cancer patients are benefiting from a new light therapy treatment which tackles a common side effect caused by chemotherapy and radiotherapy.
Photobiomodulation (PBM) has launched in hospitals run by East Suffolk and North Essex NHS Foundation Trust (ESNEFT) to prevent or treat oral mucositis. It occurs when cancer treatments damage the lining of the mouth. This can leave the tissue vulnerable to ulceration and infection.
All head and neck cancer patients having radiotherapy are being offered PBM at Colchester and Ipswich hospitals.
The treatments are being overseen by Jenny Gale, radiotherapy sister at Colchester, and Lyndsey Rew Macmillan specialist radiographer at Ipswich.
Jenny is also interim head and neck clinical nurse specialist at ESNEFT. She has had … Continue reading
Posted in Industry, PBM/LLLT, THE FUTURE OF PBM/LLLT
on New light therapy helps to ease pain for head and neck cancer patients
Pope Francis praises Light Therapy after recovery
 Pope Francis credits Light Therapy for his recovery from a wheelchair during a ceremony on Saturday, April 29, during his homily at St. Elisabeth Church in Budapest. Pope Francis spoke of how the red-light therapy healed his fractured knee. The Pope was able to stand up out of his wheelchair and walk with a and walk with a cane.
Pope Francis credits Light Therapy for his recovery from a wheelchair during a ceremony on Saturday, April 29, during his homily at St. Elisabeth Church in Budapest. Pope Francis spoke of how the red-light therapy healed his fractured knee. The Pope was able to stand up out of his wheelchair and walk with a and walk with a cane.
To read the full article, go here: https://themissionstribune.com/headlines
Posted in Industry, Special Feature
on Pope Francis praises Light Therapy after recovery
Treating children’s oral mucositis with THOR photobiomodulation
Belinda N. Mandrell PhD, RN, PNP has been at St. Jude Children’s Research Hospital for over 30 years. Belinda speaks to James Carroll about how they have been treating their oral mucositis patients with photobiomodulation.
Read more about oral mucositis
PBM RECOMMENDED FOR ORAL MUCOSITIS (NICE)
View current photobiomodulation therapy training dates in your location.
Posted in Industry, Interviews, Video of the Week
on Treating children’s oral mucositis with THOR photobiomodulation
EyewireTV News Coverage of LumiThera Trial Results
Positive New Data for LumiThera’s Valeda Light Delivery System for Dry Age-Related Macular Degeneration.
 Featured Testimonials
Featured Testimonials

