Categories
Essays
Links
Author Archives: James Carroll
The evidence for Photobiomodulation treatment of musculoskeletal pain
THERAPY EXPO SYNOPSIS: Photobiomodulation (PBM) is one of the best researched physical therapy modalities. More than 800 RCTs have been published on the benefits of PBM, thousands of papers published on the mechanism of action and dose response, more than 8,000 academic papers in total. Systematic reviews and clinical guidelines recommending PBM have been published in the worlds leading journals including The Lancet, British Journal of Sports Medicine, the British Medical Journal and JAMA for a wide range of musculoskeletal disorders. The American College of Physicians recommend PBM for Low Back Pain, and NICE recommend PBM for Oral Mucositis. PBM works primarily on the powerhouse of the cell (mitochondria), increasing energy (ATP) and reducing oxidative stress (cytokines) that lead to inflammation cell death and diseases associated with ageing. The unique feature of PBM is its ability to switch on tissue and regenerative processes. This presentation summarises the evidence for PBM in musculoskeletal pain.
For more on PBM evidence, training or … Continue reading
Posted in Research
on The evidence for Photobiomodulation treatment of musculoskeletal pain
James Carroll 2020 WALT Presidential Commendation
Thank you to The World Association for PhotobiomoduLation Therapy for the award today. As I said in my acceptance speech I am grateful for the many friends and good times I have enjoyed in the field of LLLT/PBM over the last 33 years, largely thanks to WALT. I should have said more about what I have learned from the many scientists, doctors, dentists, veterinarians, therapists, nurses and patients. It has led to an exciting, rewarding and fulfilling career, and I look forward to the next 40 years as we take PBM to where it is needed and belongs. So thank you WALT for giving me a life worth living.
Posted in James in Action, Special Feature
on James Carroll 2020 WALT Presidential Commendation
RIP Dr Kevin Moore who died last week 15 July 2020
 Kevin was a husband, father, anaesthetist, chronic pain specialist, researcher, a fun, kind and generous man.
Kevin was a husband, father, anaesthetist, chronic pain specialist, researcher, a fun, kind and generous man.
He was a vital founding member of the World Association for Laser Therapy (WALT) and a long-standing cheerleader for Photobiomodulation (PBM) as it is now known.
As well as being Consultant Anaesthetist at The Royal Oldham Hospital UK, Kevin was medical director at Dr Kershaw’s Hospice.
Kevin also had the thankless task of being the WALT treasurer for many years. His leadership and steady hand ensured the organisation survived several problematic periods.
All who met him will remember his warmth, humour and generosity of spirit.
He will be forever memorialised in my LLLT/PBM presentations as he researched and published two of my favourite PBM papers of all time (abstracts below and links to some … Continue reading
Posted in Research
on RIP Dr Kevin Moore who died last week 15 July 2020
LumiThera Enrolls First Patient in the U.S. Multi-Center, LIGHTSITE III Clinical Study to Treat Dry Age-Related Macular Degeneration
SEATTLE, Oct. 1, 2019 /PRNewswire/ — LumiThera Inc., a commercial stage medical device company delivering photobiomodulation (PBM) treatment for ocular disorders and disease, today announced it has begun enrolling patients in a multi-center United States clinical study in dry Age-Related Macular Degeneration (AMD) patients.
The randomized, multi-center study called LIGHTSITE III enrolled and treated the first patient at Cumberland Valley Retina in Hagerstown, Maryland. The United States study is being conducted in leading retinal centers throughout the United States. The study will enroll approximately 100 patients suffering from dry AMD and treat them over the course of two years. In addition to demonstrating safety, key efficacy endpoints include visual acuity, contrast sensitivity and reduction of drusen deposits.
In February, the company announced that the National Institute of Health and the division of the National Eye Institute was providing a $2.5M grant to partially support the U.S. study.
“This … Continue reading
Posted in Industry
on LumiThera Enrolls First Patient in the U.S. Multi-Center, LIGHTSITE III Clinical Study to Treat Dry Age-Related Macular Degeneration
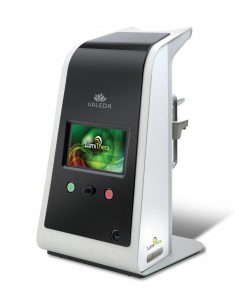
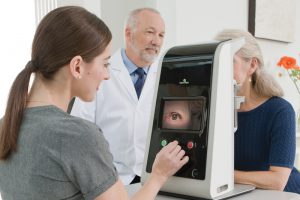
Valeda Light Delivery System Receives 2019 Medical Design Excellence Award
SEATTLE, June 12, 2019 /PRNewswire/ — LumiThera, Inc. and Product Creation Studio today announced that LumiThera’s Valeda Light Delivery System has been honored with a silver award in the 21st Annual Medical Design Excellence Awards competition. The 2019 winners were announced at the MDEA Ceremony on Tuesday, June 11, 2019 in conjunction with the MD&M East event in New York, New York.
LumiThera, Inc. is a leader in the use of photobiomodulation for the treatment of acute and chronic ocular diseases and disorders. Age-related macular degeneration (AMD) is the leading cause of blindness in adults over 65. Product Creation Studio worked with LumiThera from product concept through transfer to manufacturing to design and engineer a medical device that would accurately, safely, and effectively treat patients suffering from dry AMD. The resulting Valeda Light Delivery System is currently the only approved treatment using PBM) treatment for ocular disorders and disease, today announced it is a recipient of a small business innovative research (SBIR) phase II grant from the National Institute of Health (NIH) and the division of the National Eye Institute (NEI).

Posted in Industry
on LumiThera Receives Notice of Award for $2.5 Million National Eye Institute Grant to Support U.S. Multi-Center Clinical Trial for Treating Dry Age-Related Macular Degeneration
New Technologies, Treatments Can End Opioid Use


On Oct. 24, President Donald Trump signed the Opioid Crisis Response Act (OCRA) into law. Dozens of bills designed to address the diverse aspects of the opioid crisis were consolidated into one strategic and integrated approach.
OCRA received overwhelming bi-partisan support in both Chambers.
HR6, which is now Public Law 115-2716, broke new ground in being the first legislation to mandate aggressive development and adoption of alternative pain treatments that include “innovative medical technologies for pain management.”
On Oct. 11, Congress held its first ever briefing on ending opioid use through “innovative medical technologies for pain management.”
Photobiomodulation (PBM) was the featured technology.
Read the full news story on newsmax.com
Posted in Industry, THE FUTURE OF PBM/LLLT
on New Technologies, Treatments Can End Opioid Use
 Featured Testimonials
Featured Testimonials