Categories
Essays
Links
Category Archives: Industry
Seven News – Photobiomodulation for chronic pain
Dr Roberta Chow PhD and Lydia Feng talk about the use of light and lasers for relief of chronic pain. The THOR handheld unit and NovoTHOR light-pod can be used to reduce the need for medication and delay the need for surgery without any side-effects.
View current photobiomodulation therapy training dates in your location.
Posted in Industry, Interviews, Video of the Week
on Seven News – Photobiomodulation for chronic pain
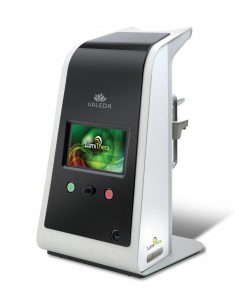
Valeda Light Delivery System Receives 2019 Medical Design Excellence Award
SEATTLE, June 12, 2019 /PRNewswire/ — LumiThera, Inc. and Product Creation Studio today announced that LumiThera’s Valeda Light Delivery System has been honored with a silver award in the 21st Annual Medical Design Excellence Awards competition. The 2019 winners were announced at the MDEA Ceremony on Tuesday, June 11, 2019 in conjunction with the MD&M East event in New York, New York.
LumiThera, Inc. is a leader in the use of photobiomodulation for the treatment of acute and chronic ocular diseases and disorders. Age-related macular degeneration (AMD) is the leading cause of blindness in adults over 65. Product Creation Studio worked with LumiThera from product concept through transfer to manufacturing to design and engineer a medical device that would accurately, safely, and effectively treat patients suffering from dry AMD. The resulting Valeda Light Delivery System is currently the only approved treatment using Photobiomodulation … If you’ve never heard of this well researched and incredible technology to decrease inflammation and shorten healing time, watch our Podcast Video! PBM has successfully helped thousands of people in over 70 countries with chronic pain, macular degeneration, shingles pain, recovery from athletic injury, CTE, PTSD and more. Check out ThorLaser.com to connect with James, his research, and how THOR PBM can improve your life.”
Gut Check Project
Kenneth Brown, MD
Board Certified Gastroenterologist
View current photobiomodulation therapy training dates in your location.
Posted in Industry, Interviews, James in Action, THE FUTURE OF PBM/LLLT, Video of the Week
on Podcast interview of James Carroll by Gut Check Project
James Carroll’s Photobiomodulation and NovoTHOR interview
Laser Therapy Sydney interviews James Carroll CEO and Founder of Thor Photomedicine and the NovoTHOR light-pod.
James Carroll sheds light on what Photobiomodulation is and what conditions it can treat; who is using it and how Thor products are different; oral mucositis and the opioid crisis; and the future of PBM in Australia.
View current photobiomodulation therapy training dates in your location.
Posted in Industry, Interviews, James in Action, THE FUTURE OF PBM/LLLT, Video of the Week
on James Carroll’s Photobiomodulation and NovoTHOR interview
Congressional Briefing Highlight Video
U.S. CONGRESSIONAL BRIEFING. Ending Opioid Use – Washington DC
James Carroll presented evidence to Congress on the effectiveness of PBM for treating pain and where it can be used in place of opioids. Prof. Praveen Arany explained how and why it works, and Annette Quinn RN gave her first hand experience in treating over 854 patients with Oral Mucositis.
Learn more at CongressPBM.com
Watch James Carroll’s Congress speech on ending opioid use with Photobiomodulation
Read James Carroll’s Speech to Congress on citizenoversight.blogspot.com
View current photobiomodulation therapy training dates in your location.
Posted in Industry, James in Action, THE FUTURE OF PBM/LLLT, Video of the Week
on Congressional Briefing Highlight Video
LumiThera Receives Notice of Award for $2.5 Million National Eye Institute Grant to Support U.S. Multi-Center Clinical Trial for Treating Dry Age-Related Macular Degeneration
LumiThera Inc., a commercial stage medical device company creating a photobiomodulation (PBM) treatment for ocular disorders and disease, today announced it is a recipient of a small business innovative research (SBIR) phase II grant from the National Institute of Health (NIH) and the division of the National Eye Institute (NEI).
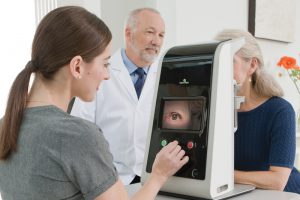 The phase II grant supports a prospective, randomized, multi-center human clinical trial in U.S. subjects diagnosed with dry age-related macular degeneration (dry AMD.) The LIGHTSITE III trial, which is subject to FDA Investigational Device Exemption (IDE) approval, will test vision and examine disease pathology in the eye following PBM treatments using the Company’s Valeda™ Light Delivery System. Subjects will be followed for up to two years. In 2018, LumiThera obtained a CE mark to commercialize the Valeda™ Light … Continue reading
The phase II grant supports a prospective, randomized, multi-center human clinical trial in U.S. subjects diagnosed with dry age-related macular degeneration (dry AMD.) The LIGHTSITE III trial, which is subject to FDA Investigational Device Exemption (IDE) approval, will test vision and examine disease pathology in the eye following PBM treatments using the Company’s Valeda™ Light Delivery System. Subjects will be followed for up to two years. In 2018, LumiThera obtained a CE mark to commercialize the Valeda™ Light … Continue reading
Posted in Industry
on LumiThera Receives Notice of Award for $2.5 Million National Eye Institute Grant to Support U.S. Multi-Center Clinical Trial for Treating Dry Age-Related Macular Degeneration
 Featured Testimonials
Featured Testimonials